SKS : 2
DOSEN : Dr. Syamsurizal, M.Si
WAKTU : 22-29 Desember 2012
PETUNJUK : Ujian ini open book. Tapi tidak diizinkan mencontek, bilamana ditemukan, maka anda dinyatakan GAGAL. Jawaban anda diposting di bolg masing-masing.
1. Jelaskan dalam jalur biosintesis triterpenoid, identifikasilah faktor-faktor penting yang sangat menentukan dihasilkannya triterpenoid dalam kuantitas yang banyak.
2. Jelaskan dalam penentuan struktur flavonoid, kekhasan signal dan intensitas serapan dengan menggunakan spektrum IR dan NMR. Berikan dengan contoh sekurang-kurangnya dua struktur yang berbeda.
3. Dalam isolasi alkaloid, pada tahap awal dibutuhkan kondisi asam atau basa. Jelaskan dasar penggunaan reagen tersebut, dan berikan contohnya sekurang-kurangnya tiga macam alkaloid.
4. Jelaskan keterkaitan diantara biosintesis, metode isolasi dan penentuan struktur senyawa bahan alam . Berikan contohnya.
answers:
1. Here is a terpenoid biosynthetic pathway
Picture from : (http://dc438.4shared.com/doc/vekXSryo/preview.html)
From the image above we can see that the triterpenoids
derived from geranil geranil-pyrophosphate (GGPP) derived from the condensation
between the units or IPP and GPP by the same mechanism. So, I think a very
important factor determining the quantity produced triterpenoids which many are
on the incorporation of IPP and GPP that produces farnesil pyrophosphate (FPP).
2. Infrared spectroscopy
Spectrophotometric Infra Red or
Infra Red is a method to observe the interaction of molecules with
electromagnetic radiation is in the region from 0.75 to 1000 μm wavelength or
wave number 13000-10 cm-1 by using a tool that Infrared Spectrophotometer.
This method is widely used in
laboratory industrial analysis and research laboratories because it can provide
useful information for qualitative and quantitative analysis, as well as assist
in the implementation of a compound of formula-up.
IR spectra are very rich in
molecular information consisting sktruktur rotational and vibrational motion.
IR spectrum lies in the range of
wave numbers 12800-10 cm-1. In terms of applications and instrumentation, IR
spectrum is divided into three types of radiation, namely the near IR, mid-,
and far away.). IR spectroscopy to qualitative analysis methods are very useful,
but difficult to do because of the similarity of each response spectrum.
Quantitative analysis of the IR spectrum is also very difficult because of the
overlap of the absorption spectra of the molecules in the sample. To be able to
extract information from the data is complex IR spectrum, required a method
kemometrik form of multivariate analysis.
NMR spectrum
Measurement of one-dimensional
NMR spectra can be used to determine the structure of molecules to simpler
compounds and is a known compound. Much information can be extracted from the
measurement result 1 dimensional NMR spectra (1H and 13C-NMR), such as the
presence of functional groups that is expressed in a typical such as the
number, type and position of functional groups, the number of protons and
carbon and can determine the shape conformation or spatial structure such as
cis or trans, axial or equatorial.
With the known functional groups
based on the measurement of 1H and 13C NMR spectra, to assemble clusters into a
molecular structure is complete and correct measurements were taken
two-dimensional NMR spectra. But to do intrepetasi Data 2D NMR spectral data
such as HMQC, HMBC, and NOE requires considerable time given the required
competence and experience Yany long enough. Besides, considering the molecular
structure varies from simple to very complex.
Example:
I.
Identification of flavonoid structure
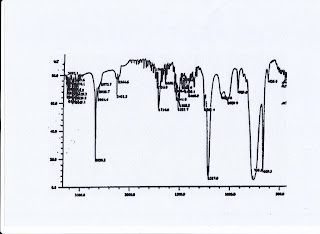
Further analysis using IR
spectrophotometer to determine the functional gugusgugus compounds are at f2
fraction is shown in Figure.
Figure of spectrum IR
I. Flavonoids in the leaves of breadfruit
1H NMR spectrum of compound 1
showed a signal at δH 12.85 ppm for the-OH terkelasi with the-C = O, two
triplet signals of two methylene groups in the δH 3.11 and 2.97 ppm for the two
methylene groups-α and -β from dihidrokalkon, and the number of signals
corresponding to one unit geranil (sinyals at δH 5.17, 5.02, 3.40, 2.08,
2.06, 1.79, 166, 1.57 ppm). In addition, the analysis of signals in the
aromatic region shows the unit 1,2,3,4 - substituted benzene (δH 6.71 and
6.65 ppm) and 2,4-gu-gus dihidroksibenzoil (δH 7.58, 6 , 37 and 6.35 ppm).
Having regard to the value of shear proton chemical signals of two aromatic
units, it was concluded that compound 1 is 2-geranil-2 ', 4', 3.4
tetrahidroksidihidrokalkon. 13C NMR data comparison between the isolated
compound 1 shows the reported high compliance. It can be concluded that
2-geranil-2 ', 4',3,4-tetrahidroksidihidrokalkon defined as compounds
3. Reagents for alkaloid compounds is wagner
reagents, reagent meyer, and dragendorf.
Some of the reagents used for the
deposition separated alkaloid. Reagent is often based on the ability to join
metal alkaloid that has a high atomic weight such as mercury, bismuth, tungsen,
or Jood. Mayer reagent containing potassium chloride and mercury jodida and
Dragendorff reagent containing bismuth nitrate and mercury chloride in aqueous
nitrite. Bouchardat reagents similar to Wagner's reagent and potassium jodida
and Jood. Menandung silikotungstat acid reagent complex silicon dioxide and
tungsten trioxide. Various reagents showed the greatest difference in alkaloid
halsensitivitas against different groups. Judging from the popularity, mayer
formulation is less sensitive than the reactants or dragendorff wagner.
Example:
a)
isolation of alkaloids, Most alkaloids are
insoluble in petroleum ether extract but selaluu should be checked for the presence of alkaloids by using one reagent
precipitating alkaloids. When the number of alkaloids soluble in petroleum
ether solvent, the plant material at the beginning coupled with aqueous
acid to bind the alkaloid salts.
b)
Isolation of nicotine from tobacco by adding
NaOH solvent
c)
Isolation of caffeine from tea leaves with
sodium carbonate solvent
4. isolation is a method to separate compounds that
mix so that we can produce a pure single compound. Plants contain thousands of
compounds that are categorized as primary metabolites and secondary
metabolites. Usually the isolation of compounds from natural ingredients are used
to isolate the secondary metabolites, as secondary metabolites can benefit
human life. Among other benefits in the areas of agriculture, health and food.
Biosynthesis is the formation of a naturally occurring
molecule in the cells of other molecules that are less complicated structure,
through endeorganik reaction. While the biosynthetic pathway can be defined as
a sequence or a process in which consists of the stages of formation of simple
compounds into complex compounds. Biosynthesis process will take place very
complex, depending on the available enzymes that similar plants growing in
different areas it is possible to have a certain metabolite formation paths are
not identical.
While the determination of the
structure performed on the isolated compound. Determining the structure of a
compound can be carried out using IR spectrum, UV, or NMR. The spectrum can
identify the shape of the structure of a compound with a specific absorption.
For example;
biosynthesis of flavonoids compound in Curcuma
aeruginosa Roxb, then in isolation to obtain a pure single compound later
identified the compound structure using IR spectrum, UV, or NMR