Acid-Base Chemistry
Water
We typically talk about acid-base reactions in aqueous-phase environments -- that is, in the presence of water. The most fundamental acid-base reaction is the
dissociation of water:
H2O  H+ + OH- H+ + OH- |
In this reaction, water breaks apart to form a hydrogen ion (H
+) and a hydroxide ion (OH
-). In pure water, we can define a special equilibrium constant (K
w) as follows:
| KW = [H+][OH-] = 1.00x10-14 |
Where K
w is the equilibrium constant for water at 25° C (unitless)
[H
+] is the molar concentration of hydrogen
[OH
-] is the molar concentration of hydroxide
An equilibrium constant less than one (1) suggests that the reaction prefers to stay on the side of the reactants -- in this case, water likes to stay as water. Because water hardly
ionizes, it is a very poor conductor of electricity.
pH
What is of interest in this reading, however, is the acid-base nature of a substance like water. Water actually behaves both like an
acid and a
base. The acidity or basicity of a substance is defined most typically by the
pH value, defined as below:
pH = -log[H+] At equilibrium, the concentration of H
+ is 1.00 × 10
-7, so we can calculate the pH of water at equilibrium as:
pH = -log[H+]= -log[1.00 × 10-7] = 7.00 Solutions with a pH of seven (7) are said to be neutral, while those with pH values below seven (7) are defined as acidic and those above pH of seven (7) as being basic.
pOH gives us another way to measure the acidity of a solution. It is just the opposite of pH. A high pOH means the solution is acidic while a low pOH means the solution is basic.
pOH = -log[OH-] pH + pOH = 14.00 at 25°C Definitions of acids and bases
- Arrhenius
acid: generates [H+] in solution
base: generates [OH-] in solution
normal Arrhenius equation: acid + base  salt + water
salt + water
example: HCl(aq) + NaOH(aq)  NaCl(aq) + H2O(l)
NaCl(aq) + H2O(l)
- Brønsted-Lowery:
acid: anything that donates a [H+] (proton donor)
base: anything that accepts a [H+] (proton acceptor)
normal Brønsted-Lowery equation: acid + base  acid + base
acid + base
example: HNO2(aq) + H2O(aq)  NO2-(aq)+ H3O+(aq)
NO2-(aq)+ H3O+(aq)
Each acid has a conjugate base and each base has a conjugate acid. These conjugate pairs only differ by a proton. In this example: NO2- is the conjugate base of the acid HNO2 and H3O+ is the conjugate acid of the base H2O.
- Lewis:
acid: accepts an electron pair
base: donates an electron pair
The advantage of this theory is that many more reactions can be considered acid-base reactions because they do not have to occur in solution.
Salts
A
salt is formed when an acid and a base are mixed and the acid releases H
+ ions while the base releases OH
- ions. This process is called
hydrolysis. The pH of the salt depends on the strengths of the original acids and bases:
| Acid | Base | Salt pH |
|---|
| strong | strong | pH = 7 |
| weak | strong | pH > 7 |
| strong | weak | pH < 7 |
| weak | weak | depends on which is stronger |
These salts are acidic or basic due to their acidic or basic ions. When weak acids or weak bases react with water, they make strong conjugate bases or conjugate acids, respectively, which determines the pH of the salt.
For a molecule with a H-X bond to be an acid, the hydrogen must have a positive
oxidation number so it can ionize to form a positive +1 ion. For instance, in sodium hydride (NaH) the hydrogen has a -1 charge so it is not an acid but it is actually a base. Molecules like CH
4 with nonpolar bonds also cannot be acids because the H does not ionize. Molecules with strong bonds (large
electronegativity differences), are less likely to be strong acids because they do not ionize very well. For a molecule with an X-O-H bond (also called an
oxoacid) to be an acid, the hydrogen must again ionize to form H
+. To be a base, the O-H must break off to form the hydroxide ion (OH
-). Both of these happen when dealing with oxoacids.
Strong Acids: These acids completely ionize in solution so they are always represented in chemical equations in their ionized form. There are only seven (7) strong acids:
HCl, HBr, HI, H2SO4, HNO3, HClO3, HClO4 To calculate a pH value, it is easiest to follow the standard "Start, Change, Equilibrium" process.
Example Problem: Determine the pH of a 0.25 M solution of HBr.
| Answer: |
| HBr (aq) | → | H+(aq) | + | Br-(aq) |
| Start: | .25 M |
| 0 M |
| 0 M |
| Change: | -.25 |
| +.25 |
| +.25 |
| Equilibrium: | 0 |
| .25 |
| .25 |
| pH = -log[H+] = -log(.25) = 0.60 |
Weak Acids: These are the most common type of acids. They follow the equation:
HA(aq)  H+(aq) + A-(aq)
H+(aq) + A-(aq) The equilibrium constant for the dissociation of an acid is known as
Ka. The larger the value of
Ka, the stronger the acid.
| Ka = | [H+][A-]

[HA] |
Example Problem: Determine the pH of 0.30 M acetic acid (HC
2H
3O
2) with the
Ka of 1.8x10
-5.
| Answer: |
| Write an equilibrium equation for the acid: |
HC2H3O2  H+ + C2H3O2- H+ + C2H3O2- |
| Write the equilibrium expression and the Ka value: |
| Ka = | [H+][C2H3O2-]

[HC2H3O2] | = 1.8x10-5 |
|
| "Start, Change, Equilibrium": |
| HC2H3O2 |  | H+ | + | C2H3O2- |
| Start: | 0.30 M |
| 0 M |
| 0 M | |
| Change: | -x |
| +x |
| +x |
| Equilibrium: | 0.30 - x |
| x |
| x |
|
Substitute the variables (disregard the "-x" because it is so small compared to the 0.30)
and solve for [H+]: |
| Ka = 1.8x10-5 = | (x)(x)

(.30 - x) | = | x2

.30 |
|
| x = [H+] = 2.3x10-3 |
| pH = -log[H+] = 2.64 |
Strong Bases: Like strong acids, these bases completely ionize in solution and are always represented in their ionized form in chemical equations. There are only eight (8) strong bases:
LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH)2, Sr(OH)2, Ba(OH)2 Example Problem: Determine the pH of a 0.010 M solution of Ba(OH)
2.
| Answer: |
| Ba(OH)2(aq) | → | Ba2+(aq) | + | 2OH-(aq) |
| Start: | .010 M |
| 0 M |
| 0 M |
| Change: | -.010 |
| +.010 |
| +.010 |
| Equilibrium: | 0 |
| .010 |
| .010 |
| pOH = -log[OH-] = -log(.010) = 1.70 |
| pH = 14.00 - 1.70 = 12.30 |
Weak Bases: These follow the equation:
Weak Base + H2O  conjugate acid + OH- example: NH3 + H2O
conjugate acid + OH- example: NH3 + H2O  NH4+ + OH- Kb
NH4+ + OH- Kb is the base-dissociation constant:
| Kb = | [conjugate acid][OH-]

[weak base][H2O] |
| example: Kb = | [NH4+][OH-]

[NH3[H2O] |
Ka x Kb = Kw = 1.00x10-14 To calculate the pH of a weak base, we must follow a very similar "Start, Change, Equilibrium" process as we did with the weak acid, however we must add a few steps.
Example Problem: Determine the pH of 0.15 M ammonia (NH
3) with a
Kb=1.8x10
-5.
| Answer: |
| Write the equilibrium equation for the base: |
NH3 + H2O  NH4+ + OH- NH4+ + OH- |
| Write the equilibrium expression and the Kb value: |
| Kb = | [NH4+][OH2-]

[NH3][H2O] | = 1.8x10-5 |
|
| "Start, Change, Equilibrium": |
| NH3 | + | H2O |  | NH4+ | + | OH- |
| Start: | 0.15 M |
| -- |
| 0 M |
| 0 M | |
| Change: | -x |
| -- |
| +x |
| +x |
| Equilibrium: | 0.15 - x |
| -- |
| x |
| x |
|
Substitute the variables (disregard the "-x" because it is so small compared to the 0.15)
and solve for [OH-]: |
| Kb = 1.8x10-5 = | (x)(x)

(.15 - x) | = | x2

.15 |
|
| x = [OH-] = 1.6x10-3 M |
| pOH = -log[OH-] = 2.80 |
| pH = 14.00 - 2.80 - 11.20 |
When dealing with weak acids and weak bases, you also might have to deal with the "common ion effect". This is when you add a salt to a weak acid or base that contains one of the ions present in the acid or base. To be able to use the same process to solve for pH when this occurs, all you need to change are your "start" numbers. Add the
molarity of the ion, which comes from the salt, and then solve the
Ka or
Kb equation as you did earlier.
Example Problem: Find the pH of a solution formed by dissolving 0.100 mol of HC
2H
3O
2 with a
Ka of 1.8x10
-8 and 0.200 mol of NaC
2H
3O
2 in a total volume of 1.00 L.
| Answer: |
| HC2H3O2(aq) | → | H+(aq) | + | C2H3O2-(aq) |
| Start: | .10 M |
| 0 M |
| .20 M |
| Change: | -x |
| +x |
| +x |
| Equilibrium: | .10 - x |
| x |
| .20 + x |
| Ka = 1.8x10-8 = | (x)(.20 + x)

(.10 - x) | = | (x)(.20)

(.10) |
|
| x = [H+] = -9.0x10-9 |
| pH = -log(9.0x10-9) = 8.05 |
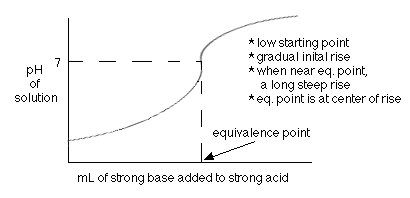
An acid-base titration is when you add a base to an acid until the equivalence point is reached which is where the number of moles of acid equals the number of moles of base. For the titration of a strong base and a strong acid, this equivalence point is reached when the pH of the solution is seven (7) as seen on the following titration curve:
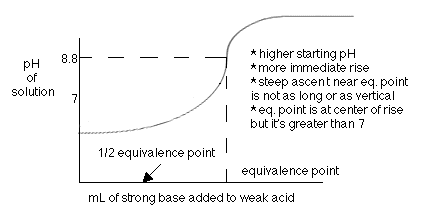
For the titration of a strong base with a weak acid, the equivalence point is reached when the pH is greater than seven (7). The half equivalence point is when half of the total amount of base needed to neutralize the acid has been added. It is at this point where the pH = p
Ka of the weak acid.
In an acid-base titration, the base will react with the weak acid and form a solution that contains the weak acid and its conjugate base until the acid is completely gone. To solve these types of problems, we will use the
Ka value of the weak acid and the molarities in a similar way as we have before. Before demonstrating this way, let us first examine a short cut, called the
Henderson-Hasselbalch Equation. This can only be used when you have some acid and some conjugate base in your solution. If you only have acid, then you must do a pure K
a problem and if you only have base (like when the titration is complete) then you must do a K
b problem.
| pH = pKa + log | [base]

[acid] |
Where:
pH is the log of the molar concentration of the hydrogen
p
Ka is the equilibrium dissociation constant for an acid
[base] is the molar concentration of a basic solution
[acid] is the molar concentration of an acidic solution
Example Problem: 25.0 mL of 0.400 M KOH is added to 100. mL of 0.150 M benzoic acid, HC
7H
5O
2 (
Ka=6.3x10
-5). Determine the pH of the solution.
| Answer: |
| Determine where in the titration we are: |
| 0.400 M x 0.025 L = 0.0100 mol KOH added |
| 0.150 M x 0.100 L = 0.0150 mol HC7H5O2 originally |
because only 0.100 mol of base has been added, that means the thitration is not
complete; this means there are two ways to solve this problem: the normal way
and the way using the Henderson-Hasselbalch Equation. |
| Normal way: |
| HC7H5O2 | + | OH- | 
| C7H5O2- | + | H2O |
| before reaction: | 0.015 mol |
| 0.0100 mol |
| 0 mol |
| -- |
| change: | -0.0100 |
| -0.0100 |
| +0.0100 |
| -- |
| after reaction: | 0.0050 |
| 0 |
| 0.0100 |
| -- |
|
| Ka = | [H+][C7H5O2-]

[HC7H5O2] | = 6.3x10-5 = | (x)(0.0100)

0.0050 |
|
| x = [H+] = 3.2x10-5 M |
| pH = -log(3.2x10-5) = 4.49 |
| Henderson-Hasselbalch Way: |
| [HC7H5O2] = | 0.0050 mol

0.125 L | = 0.040 M |
|
| [C7H5O2-] = | 0.0100 mol

0.125 L | = 0.0800 M |
|
| pH = pKa + log | [base]

[acid] |
|
| pH = -log(6.3x10-5) + log | 0.0800

0.0400 | = 4.20 + 0.30 = 4.50 |
|
This equation is used frequently when trying to find the pH of
buffer solutions. A buffer solution is one that resists changes in pH upon the addition of small amounts of an acid or a base. They are made up of a conjugate acid-base pair such as HC
2H
3O
2/C
2H
3O
2- or NH
4+/NH
3. They work because the acidic species neutralize the OH
- ions while the basic species neutralize the H
+ ions. The buffer capacity is the amount of acid or base the buffer can neutralize before the pH begins to change to a significant degree. This depends on the amount of acid or base in the buffer. High buffering capacities come from solutions with high concentrations of the acid and the base and where these concentrations are similar in value.
salt + water
NaCl(aq) + H2O(l)
acid + base
NO2-(aq)+ H3O+(aq)

